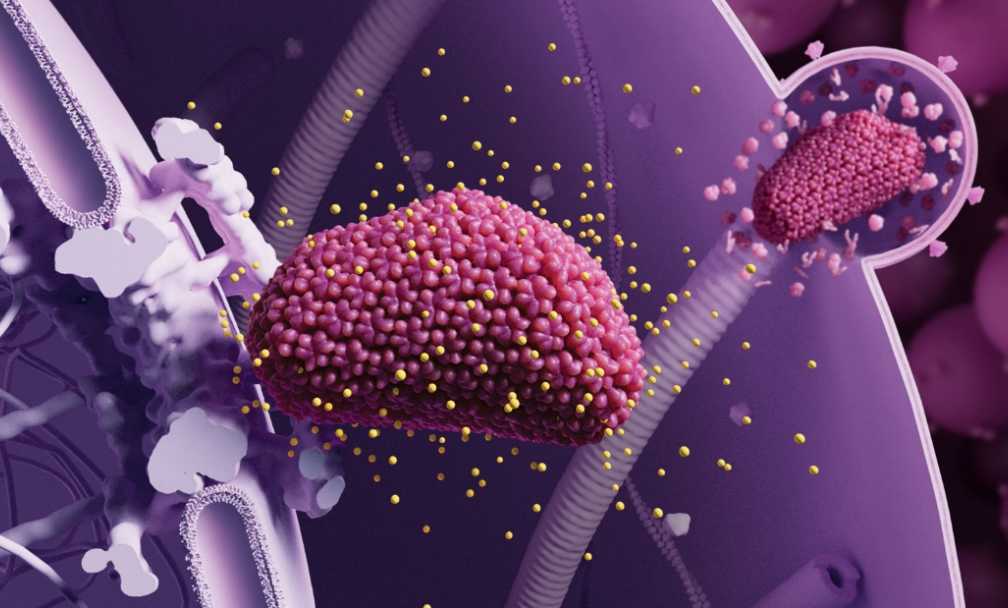
On June 20, U.S. biopharmaceutical company Gilead announced that Lenacapavir, a once - every - six - months injectable HIV - 1 capsid inhibitor, demonstrated 100% effectiveness in preventing HIV infection. This breakthrough has the potential to revolutionize HIV prevention strategies.
Gilead's HIV Drug Lenacapavir Shows 100% Efficacy in Prevention
On June 20, U.S. biopharmaceutical company Gilead announced that Lenacapavir, a once - every - six - months injectable HIV - 1 capsid inhibitor, demonstrated 100% effectiveness in preventing HIV infection. This breakthrough has the potential to revolutionize HIV prevention strategies.
Source: Images from the Internet, if there is any infringement, please contact the removal of
According to Science, the success of Lenacapavir can be attributed to significant advancements in basic research. Scientists have gained new and in - depth insights into the structure and function of the HIV capsid protein, which the drug targets. Since many viruses also possess capsid proteins, the successful application of Lenacapavir suggests that similar capsid inhibitors could potentially be developed to combat other viral diseases, opening up new avenues for antiviral treatment.
However, regulatory approval for Lenacapavir is not expected until mid - 2025 at the earliest, and its pricing remains undisclosed. As such, it remains uncertain whether this drug can accelerate the end of the AIDS epidemic. Jeanne Marrazzo, Director of the U.S. National Institute of Allergy and Infectious Diseases, cautioned that Lenacapavir cannot replace vaccines. While the drug offers promising results, a comprehensive approach involving vaccination, education, and other preventive measures is still crucial in the fight against HIV.